
Water is an extraordinary molecule containing an abundance of invisible and essential life sustaining elements. Within a single drop of water, an astrologically large number “sextrillion or 1.67 x1021” of molecules exists. The size of the tiny water molecule is approximately 0.275 nanometers or 2.75 Angstrom (x 10-10m). Water is the most abundant substance on earth.
 Water comprises a vast mixture of elements (solids, liquid & gas) derived from the earth, atmosphere and the universe. The tiny water molecules are in constant communication with each other, and with the environment. Water can occur as a nanoscale water droplet, ice crystal, invisible vapour, or be as large as the ocean. Water is as old as the universe and is constantly recycled and transformed from ice, liquid and vapour.
Water comprises a vast mixture of elements (solids, liquid & gas) derived from the earth, atmosphere and the universe. The tiny water molecules are in constant communication with each other, and with the environment. Water can occur as a nanoscale water droplet, ice crystal, invisible vapour, or be as large as the ocean. Water is as old as the universe and is constantly recycled and transformed from ice, liquid and vapour.
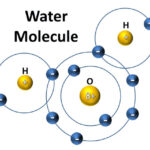
 The composition of water relates to the quality of the earth and air through which it passes. For example, acid rain or contaminated soil can change the quality of rain and the stormwater. The water molecules can penetrate, erode and dissolve materials over time, such as the rock, soil, salts, and our built environment. Water is a very reactive molecule, and known to be a universal solvent. The molecule is electrically neutral, is V-shaped, and comprises two gases with the molecular formula H2O. Water is much smaller than almost all other atmospheric molecules.
The composition of water relates to the quality of the earth and air through which it passes. For example, acid rain or contaminated soil can change the quality of rain and the stormwater. The water molecules can penetrate, erode and dissolve materials over time, such as the rock, soil, salts, and our built environment. Water is a very reactive molecule, and known to be a universal solvent. The molecule is electrically neutral, is V-shaped, and comprises two gases with the molecular formula H2O. Water is much smaller than almost all other atmospheric molecules.
 Pure liquid water (just H2O molecules) only exists in computer simulations, due to the reactivity of water in nature. On our earth, water comprises many of the “periodic table of elements”. It is a matter of how much magnification is applied for detection (e.g. from percentages (%) like Sodium or Chloride, or parts per trillion or sextrillion for metals in sea water). Water not only flows around the ocean in conveyor-belt like currents, but also in the microscopic electro-magnetic exchanges between molecules.
Pure liquid water (just H2O molecules) only exists in computer simulations, due to the reactivity of water in nature. On our earth, water comprises many of the “periodic table of elements”. It is a matter of how much magnification is applied for detection (e.g. from percentages (%) like Sodium or Chloride, or parts per trillion or sextrillion for metals in sea water). Water not only flows around the ocean in conveyor-belt like currents, but also in the microscopic electro-magnetic exchanges between molecules.
Analytical instruments can measure the natural elements & chemical impacts in our water.
 With modern analytical instruments, scientist can understand the nature of the physical, chemical and biological imprints in water through space and time. For example, sea water is saline and different to icebergs, rain (fresh water) and aquifers (geological influences) due to the various processes. From the weathering of rocks, soil and to the decay of a city, the water erodes and dissolves materials into the water ways and ocean. Water can carry substantial loads within a flowing stream such as invisible chemical concentrations (fertilisers, microplastics, oils, heavy metals etc) and visible particles and debris (litter and dusts from industry, cars, roads etc).
With modern analytical instruments, scientist can understand the nature of the physical, chemical and biological imprints in water through space and time. For example, sea water is saline and different to icebergs, rain (fresh water) and aquifers (geological influences) due to the various processes. From the weathering of rocks, soil and to the decay of a city, the water erodes and dissolves materials into the water ways and ocean. Water can carry substantial loads within a flowing stream such as invisible chemical concentrations (fertilisers, microplastics, oils, heavy metals etc) and visible particles and debris (litter and dusts from industry, cars, roads etc).
 Water sustains everything on the planet from the weathering of minerals to soil, growth of plants, animals and the also transfer of genetic information. It should be widely known that water pollution occurs very easily, as even relatively inert materials can release many toxins (e.g. Pb, Cd, benzene from cars and treated/painted timber, plastic) of which can stunt the growth of humans, plants and animals. The regulatory guidelines for many of our common pollutants (e.g. lead, benzene) are provided in low concentrations, such as in parts per billion (ppb).
Water sustains everything on the planet from the weathering of minerals to soil, growth of plants, animals and the also transfer of genetic information. It should be widely known that water pollution occurs very easily, as even relatively inert materials can release many toxins (e.g. Pb, Cd, benzene from cars and treated/painted timber, plastic) of which can stunt the growth of humans, plants and animals. The regulatory guidelines for many of our common pollutants (e.g. lead, benzene) are provided in low concentrations, such as in parts per billion (ppb).
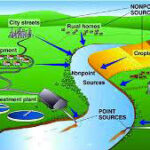 The lessons I’ve learnt over the past 30 years with site investigations is the need for better waste management and education to limit the toxic chemical impacts to the earth and vulnerable water ways. Water remediation and protection needs to include many aspects; community engagement (e.g. education, eco-friendly products, planning), engineering solutions (e.g. stormwater management with macro & micro-filtering capability), and government incentives (e.g. re-cycling, and re-use programs).
The lessons I’ve learnt over the past 30 years with site investigations is the need for better waste management and education to limit the toxic chemical impacts to the earth and vulnerable water ways. Water remediation and protection needs to include many aspects; community engagement (e.g. education, eco-friendly products, planning), engineering solutions (e.g. stormwater management with macro & micro-filtering capability), and government incentives (e.g. re-cycling, and re-use programs).
Keeping the tiny water molecule free of harmful pollutants is likely to become our greatest challenge for our generation and the next.
- 19 August 2020
- Water
